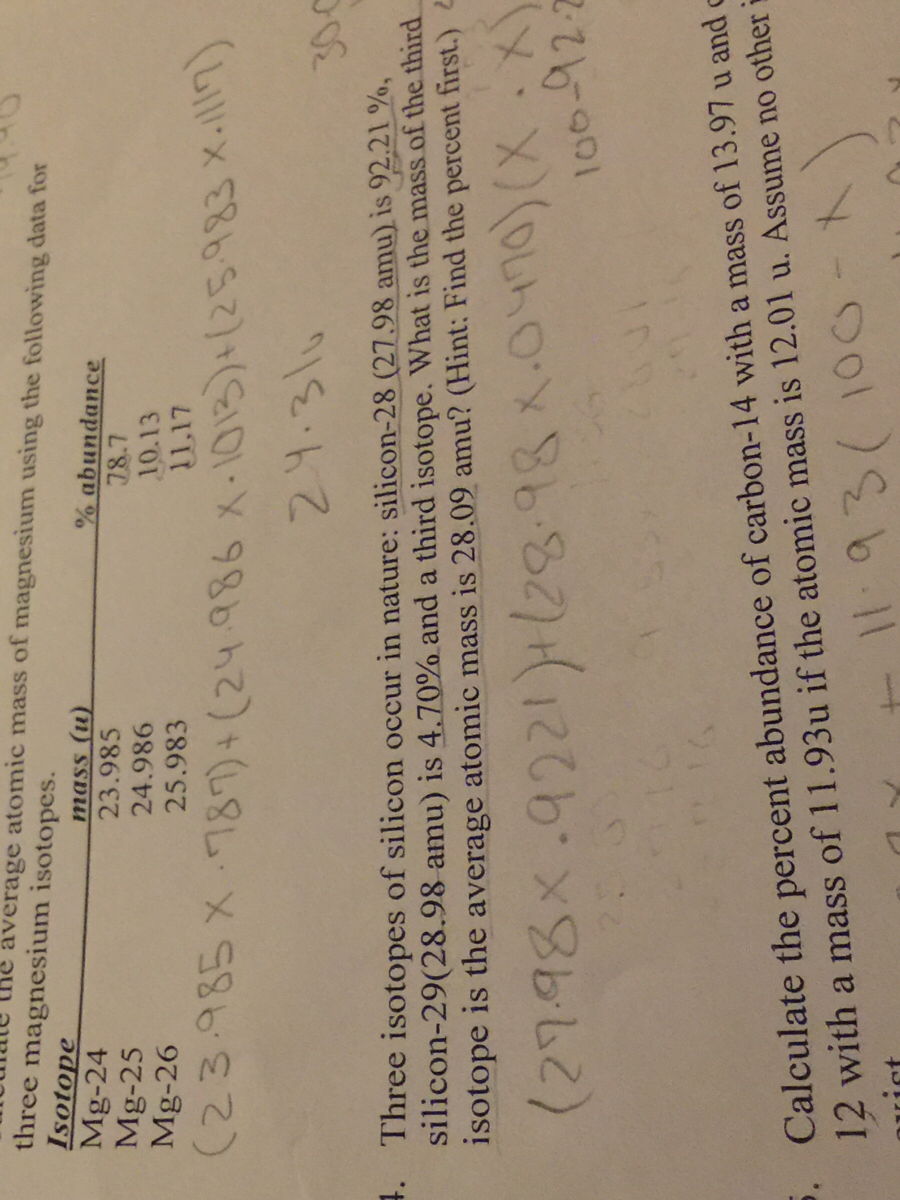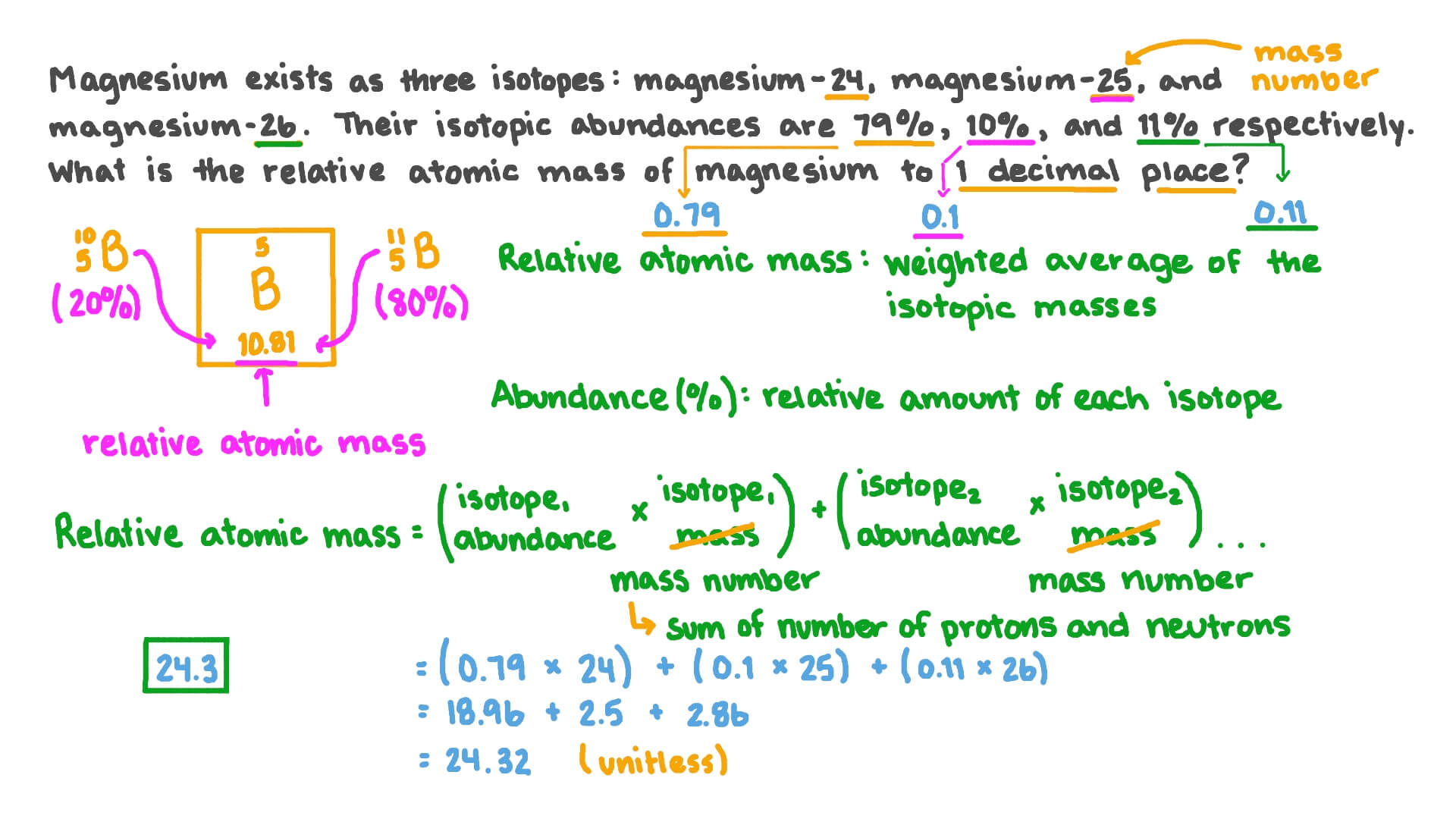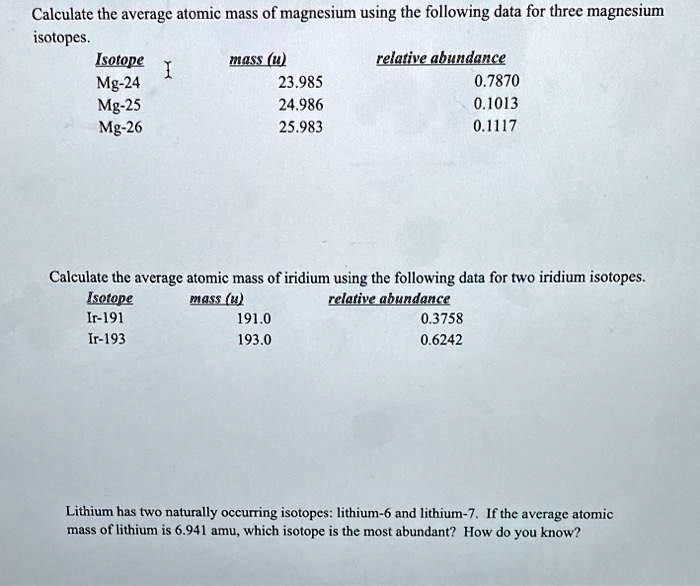
SOLVED: Calculate the average atomic mass of magnesium using the following data for threc magnesium Isotopes Ksotope massl relativg abuudance Mg-24 23.985 0.7870 Mg-25 24.986 0.1013 Mg-26 25,983 0.1417 Calculatc the average

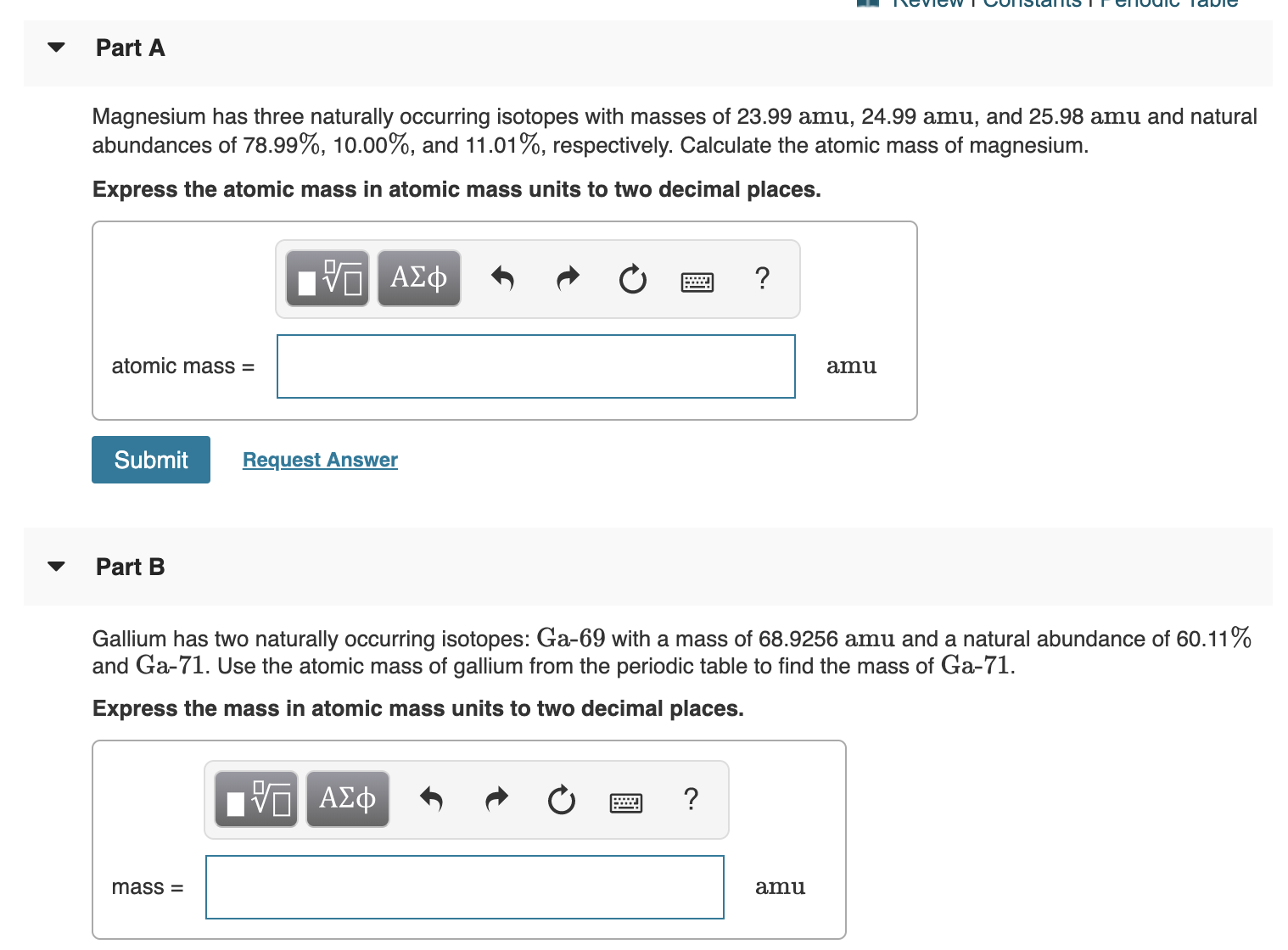
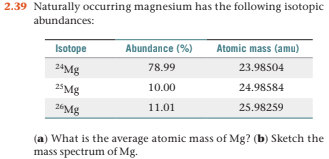
Magnesium has three naturally occurring isotopes with the following masses and natural abundances: \begin{array}{|c|c|c|} \hline \text{Isotope} & \text{Mass (amu)} & \text{Abundance (%)} \\ \hline \text{Mg-24} & \text{23.9850} & \text{78.99} \\ \hline \t
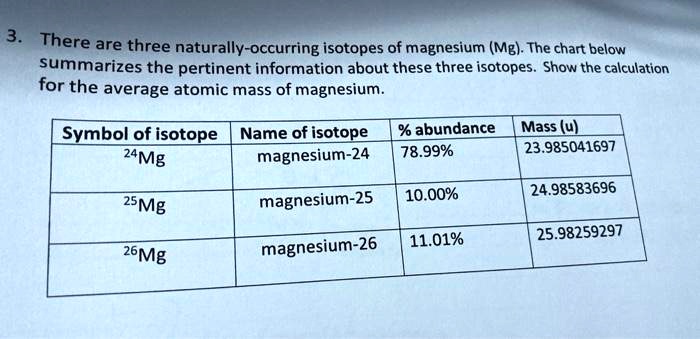
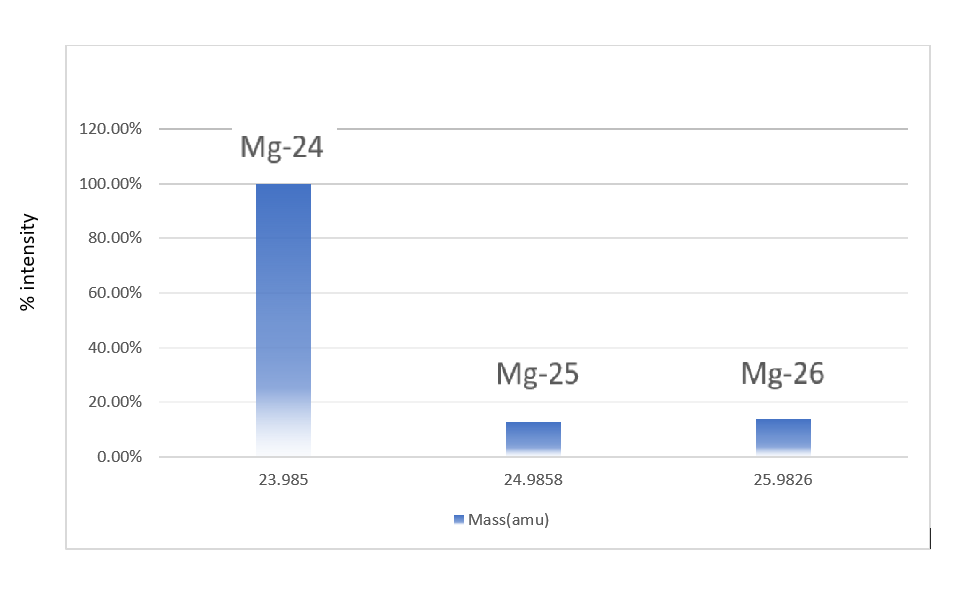
SOLVED: There are three naturally-occurring isotopes of magnesium (Mg) The chart below summarizes the pertinent information about these three isotopes Show the calculation for - the average atomic mass of magnesium. Symbol

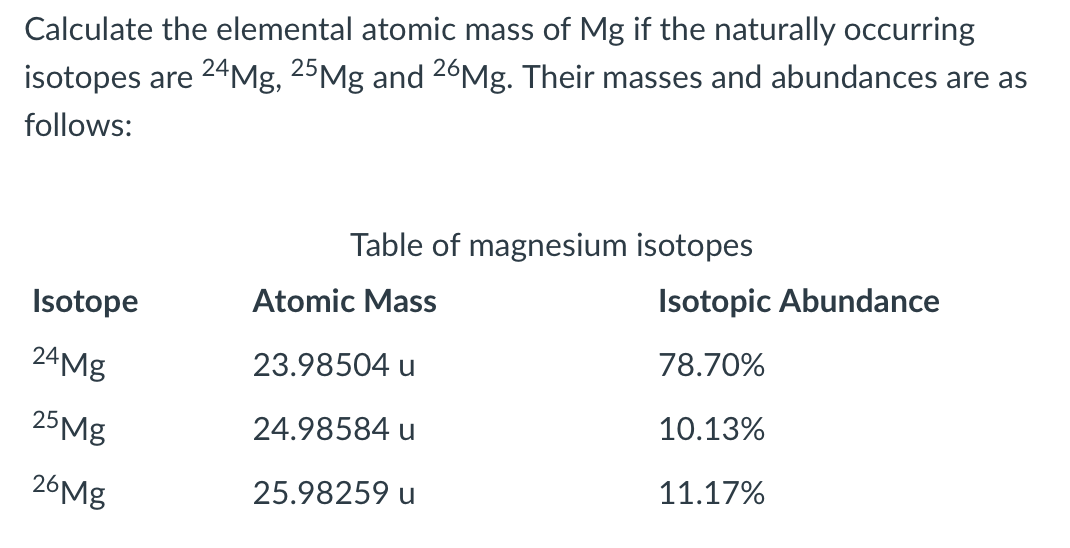
In a periodic table the average atomic mass of magnesium is given as 24.312 u. The average value is based on their relative natural abundance on earth. The three isotopes and their

In a periodic table, the averge atomic mass of magnesium is given as 24.312 u. The average value is based on their relative natural abundance on earth. The three isotopes and their

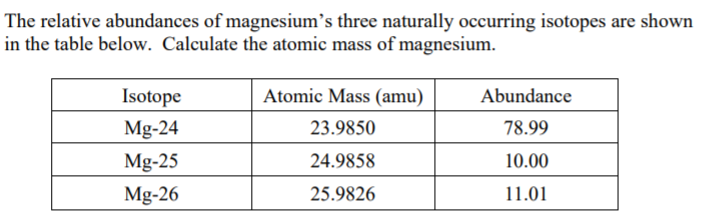
SOLVED: Naturally occurring magnesium has an atomic mass of 24.312 and consists of three isotopes. The major isotope is 24Mg, natural abundance 78.99%, relative atomic mass 23.98504. The next most abundant isotope